Chemistry 11
Unit #3 - Elements, Compounds And Mixtures
Section #1 - The Classification Of Matter
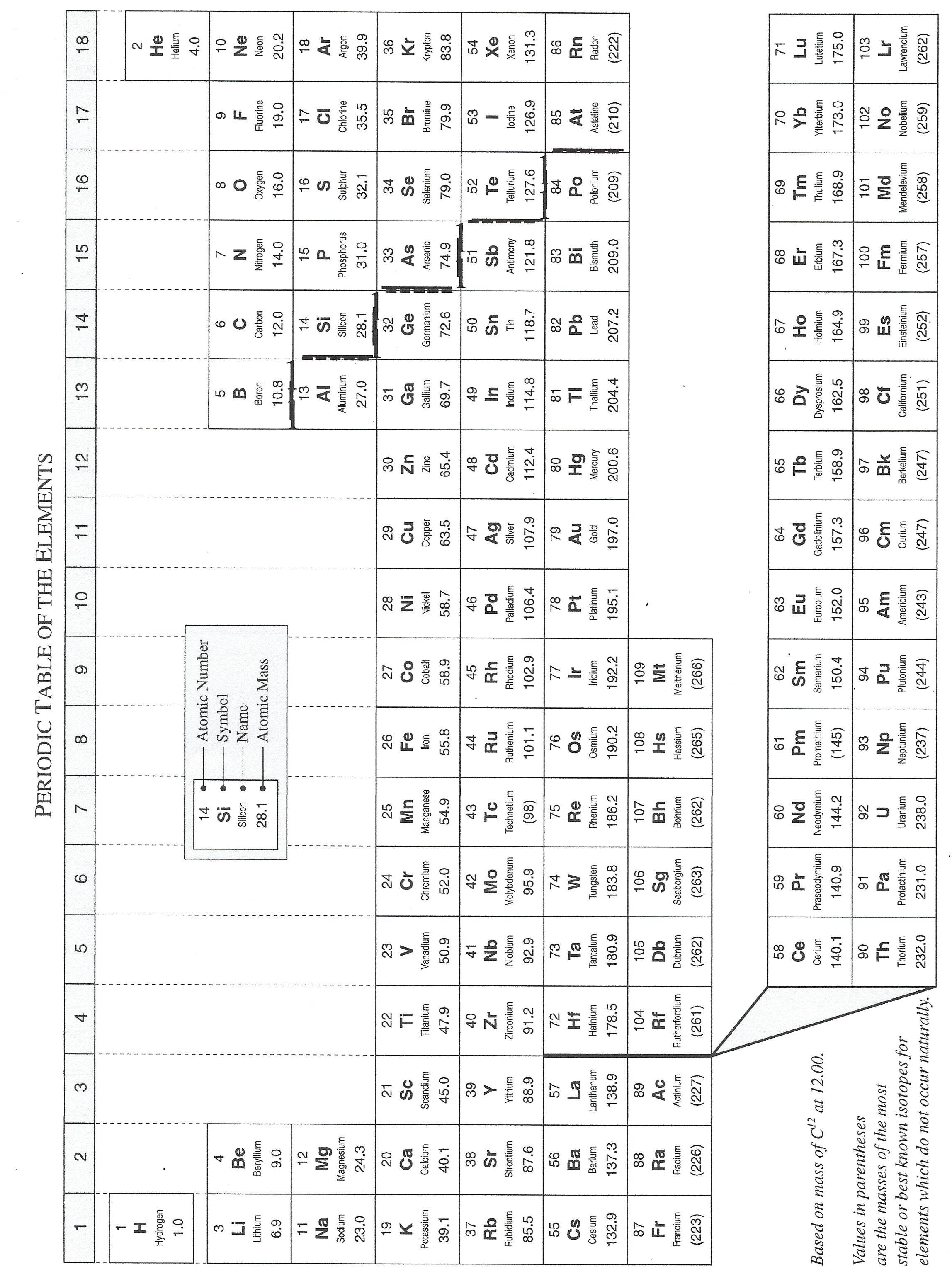
Matter is made of atoms. There are only 118 different types of atoms, but millions of possible ways to combine them. That is why there are millions of different types of substances.
The Periodic Table lists all of the known types of atoms. To see this table, click on the image to the left.
*** Don't forget to print off a copy of the Periodic Table. ***
In this version of the Periodic Table, the atomic masses have been rounded off to the first decimal place. Other versions of the Table are more precise. On our Periodic Table, Hydrogen has been given an atomic mass of 1.0. Other tables state that Hydrogen's atomic mass is 1.008.
This version of the Periodic Table is a little bit out of date. It only shows 109 different elements. In recent years, scientists have created nine new ones. These new atoms are very rare and unstable. So for all practical purposes, we don't need to know about them.
Pure Elements

Atoms are the building blocks of matter. If a substance is only made of one type of atom, then it is called a Pure Element. Your Periodic Table lists 109 different types of atoms. Sodium is a well known element. It can be found in the left-most column of the Table.
On the left side of this page, you can see a copy of Sodium's square.
This element square tells us some important imformation about sodium. At the top of the square there is a simple whole number. This is the atom's Atomic Number. Sodium's atomic number is 11. The atomic number tells us the number of protons and electrons. Therefore each sodium atom always has 11 protons and 11 electrons.
The Element Symbol is located just below the atomic number. Sodium's element symbol is 'Na'. The element symbol is used to represent sodium in chemical formulas. All element symbols have either one or two letters. The first letter is always upper case (capital), the second letter is always lower case. The 'Na' for sodium, the 'O' for oxygen, and the 'H' for Hydrogen all follow this two letter rule.
Below the element symbol, one can see the element's name. At the very bottom of the square we can see the Atomic Mass. This tells us how massive (heavy) the atom is. According to the Periodic Table, the average Sodium atom has a mass of 23.0 u.
'u' stands for atomic mass units.
.
Atoms Are Made of Smaller Parts

The atom is the basic chemical unit of an element. However it can be broken down into smaller parts - the subatomic particles.
By clicking on the image to the left, you will be able to download a Bohr Diagram of a sodium atom.
The Nucleus is the centre of the atom. In this Bohr diagram it has been coloured yellow. It contains two types of relatively heavy subatomic particles - Protons and Neutrons.
Each proton has a charge of +1. Its atomic mass is close to 1.0 u .
Each neutron has a charge of zero. Its atomic mass is close to 1.0 u .
The outer layers are called Shells. They contain a third type of particle, the Electron. In this diagram, the electrons are represented by small green circles. Each electron has a mass of only 0.000549 u . However it has a full charge of -1.
The nucleus of this sodium atom contains 11 protons and 12 neutrons. They are closely packed together. Although it has a very small volume, the nucleus contains the overwhelming majority of the atom's mass. Each proton and neutron weighs close to 1.0 u . All sodium atoms contain 11 protons, and most of them also contain 12 neutrons. 11 plus 12 equals 23. Therefore most sodium atoms have a mass of about 23.0 u . The tiny electrons contribute very little to the atom's mass.
The Sodium atom contains three shells. The first shell, the one closest to the nucleus, only has room for two electrons. The second shell is further out, so there is space for up to eight electrons. Thus the first two shells can hold a total of 10 electrons. As a result, sodium's 11th electron is forced to go out to the third shell.
The Chemical Formula
A chemical formula uses element symbols. This shows the reader what types of atoms can be found in each type of substance. A Pure Element contains only one type of atom.
For example, pure sodium has a chemical formula of Na. Since there is only one element symbol (Na), then sodium must be a pure element.
Pure oxygen is also a pure element. Its chemical formula is O2. The formula tells us that it only contains one type of atom, namely oxygen. However, because the formula is O2, this means that the basic particle of oxygen is a molecule. It is a molecule that contains TWO oxygen atoms that have joined together.
A Molecule is a particle that contains two or more connected atoms.
The chemical formula of water is H2O . So water is also made of molecules. Each water molecule always contains two hydrogen (H) atoms and one oxygen (O) atom.
Water contains MORE than one type of atom. Therefore it is NOT a pure element.
A Compound is a substance that was made by combining two or more different types of atoms. Water is an example of a compound.
Compounds
A compound is a substance that contains two or more different elements. These elements combine at the level of the atoms. The result is either an Ionic Compound or a Molecular Compound.
How Ionic Compounds Are Formed
The zig-zag line on the Periodic Table divides the elements into metals and non-metals. Metals can be found to the left of this line, non-metals to the right.
Hydrogen is a strange element. Although it is usually shown on the left side of the Periodic Table, it is actually a non-metal.
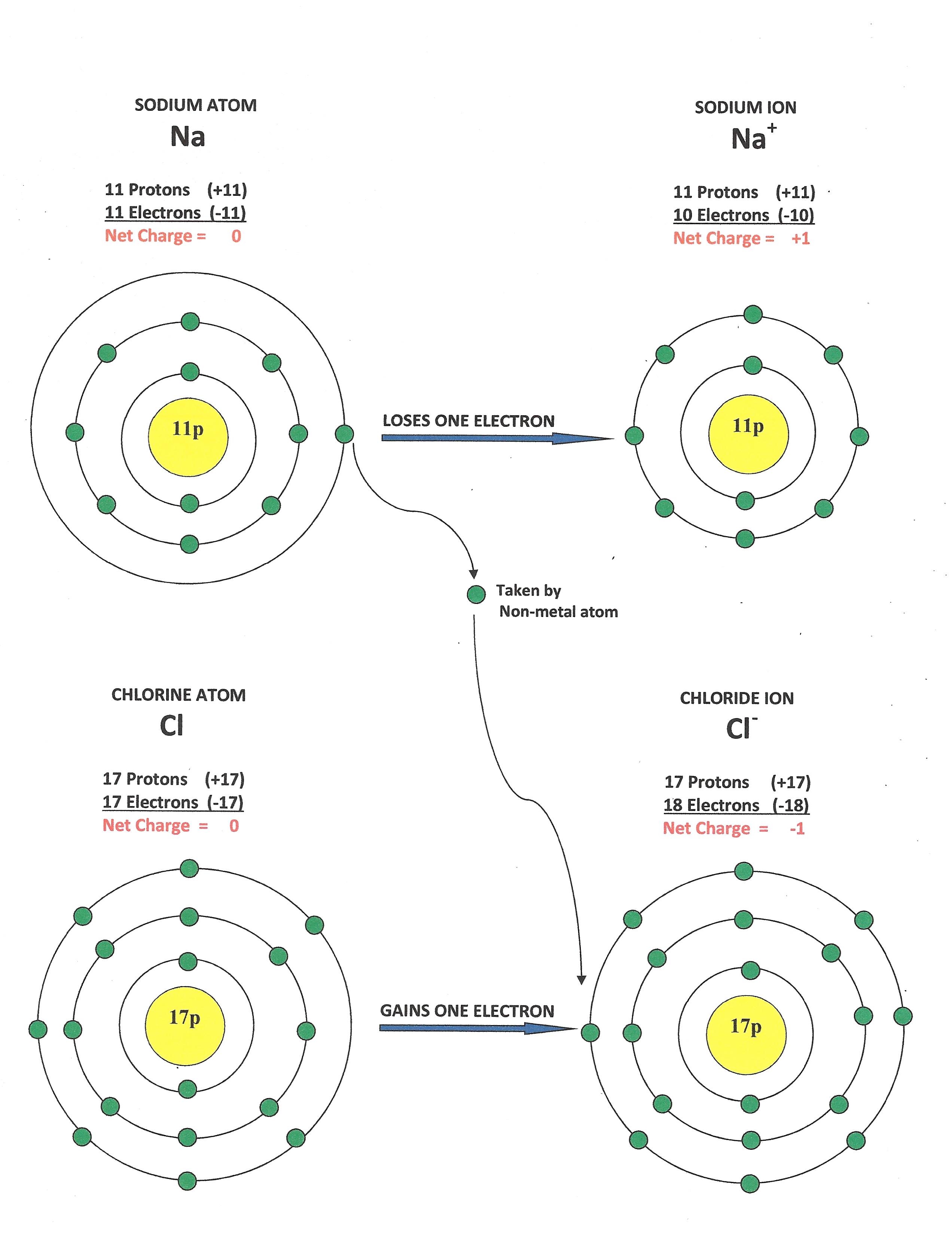
** Click on the diagram to the left. It shows how sodium reacts with chlorine. **
It would be a good idea to print off a copy of this diagram.
*** Ionic Compounds are formed when metal atoms give electrons to non-metal atoms. ***
*** IMPORTANT POINT - Only electrons move during chemical reactions. The protons and neutrons always stay inside the nucleus. In fact it is only the electrons of the outermost shell that actually take part in chemical reactions.
.
.
Why Atoms Give Or Take Electrons
All atoms want to be like those in Column #18 - the rightmost column of the Periodic Table. Column #18 elements are very stable, because they have just the right number of electrons to fill their shells. Therefore all other atoms will try to gain or lose electrons, in order to be more like the nearest Column #18 atom.
Sodium has an atomic number of 11. This means that it normally has 11 electrons. Neon in column #18 has only 10 electrons. Therefore a sodium atom will try to get rid of its one outermost electron. By doing this, it will become more like neon.
Look at the diagram and you can see that the loss of the electron turns the Sodium Atom into the Sodium Ion. An electron has a -1 charge. Therefore its loss will make sodium positive. The sodium ion always has a charge of +1.
An Ion is an atom that has a net charge.
Metal atoms lose electrons and turn into positive ions. Non-metal atoms gain electrons and turn into negative ions. The non-metal atoms are closer to Column #18, than the metals are. Therefore it is easier for the non-metals to gain electrons, instead of losing them.
A chlorine atom has an atomic number of 17. Therefore it usually has 17 electrons. This is one less than Argon in Column #18. So not surprisingly, a chlorine atom will try to take one electron. The result is the Chloride Ion. This ion always has a charge of -1.
Metals always form positive ions. Non-metals always form negative ions.
*** IMPORTANT POINT - Atoms cannot react on their own. They have to find a partner. Sodium was successfully able to give away an electron, because chlorine was willing to take it.
Some elements are very predictable. All Column #1 atoms (e.g. Sodium) need to lose one electron, to become like Column #18. As a result, all Column #1 ions have a charge of +1.
Other reliable ions include those from Column #2 (always +2), Column #17 (always -1), Column #16 non-metals (always -2) and Column #15 non-metals (always -3).
Opposites Attract
In the previous diagram, we saw that the transfer of an electron turned the sodium and chlorine atoms into the sodium and chloride ions.
Like tiny magnets, the positive ions are attracted to the negative ions. As soon as the Na+ and Cl- are formed, they stick together. This creates the Ionic Compound NaCl.
The Chemical Formula of NaCl tells us that each crystal of NaCl always has a one to one ratio of Na+ and Cl- ions. Ionic Compounds are NOT true molecules. They are simply ions that have stuck together, because opposite charges attract.
An Ionic Bond is the attraction between a positive and a negative ion.
Ionic Compounds are also called salts.
Molecular Compounds
Ionic compounds are usually combinations of metal and non-metal atoms. Molecular Compounds only contain non-metals.
Molecular Compounds never contain ions.
By looking at the chemical formulas, you should be able to recognize them. H2O only contains non-metal atoms. Therefore it is a molecular compound.
On the other hand, CaS is an ionic compound. It has both a metal (Ca) and a non-metal (S).
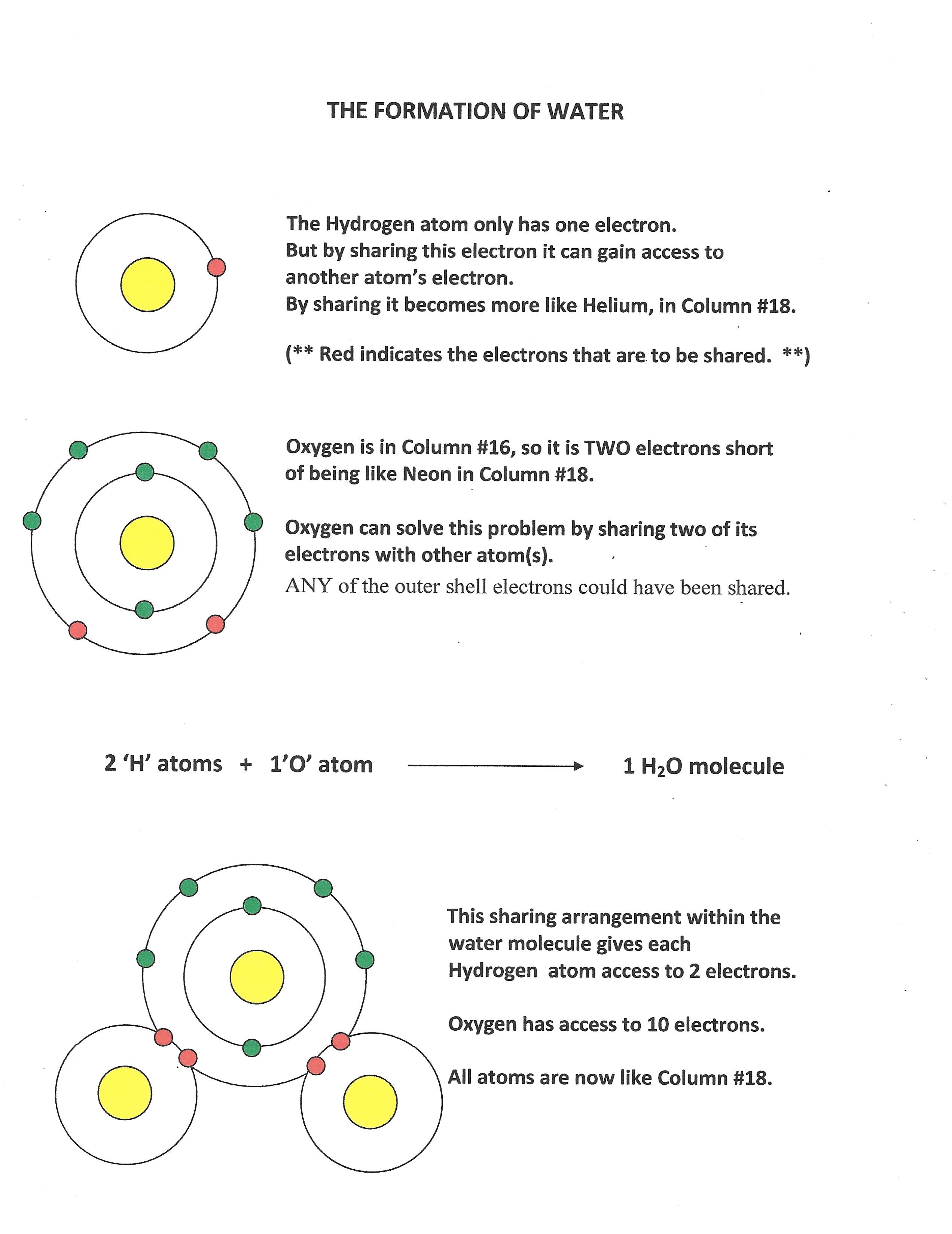
Molecular Compounds form when non-metal atoms SHARE electrons. Each time an atom shares one of its electrons, it gains access to another atom's electron. These atoms will keep on sharing until they both gain enough electrons to become like the Column #18 elements.
Click on the diagram to the left. It explains how electrons are shared within a water molecule.
None of the atoms really gain or lose electrons. But by sharing, all of them can pretend that they have gained.
A Covalent Bond is a pair of electrons shared by two atoms.
In this diagram, one can see that the oxygen atom shares a pair of electrons with each hydrogen atom. In other words, one covalent bond forms between each hydrogen atom and oxygen.
.
.
The Law Of Definite Composition
All compounds are made of specific proportions of specific elements. For example water is always made of H2O molecules. Always, there are two hydrogen atoms for each oxygen atom. Even if the chemical formula changes by a single atom, we will end up with a completely different substance. For example, H2O2 is NOT water. It is in fact Hydrogen Peroxide.
Sucrose (table sugar) is always C12H22O11. Change the chemical formula, and you no longer have table sugar.